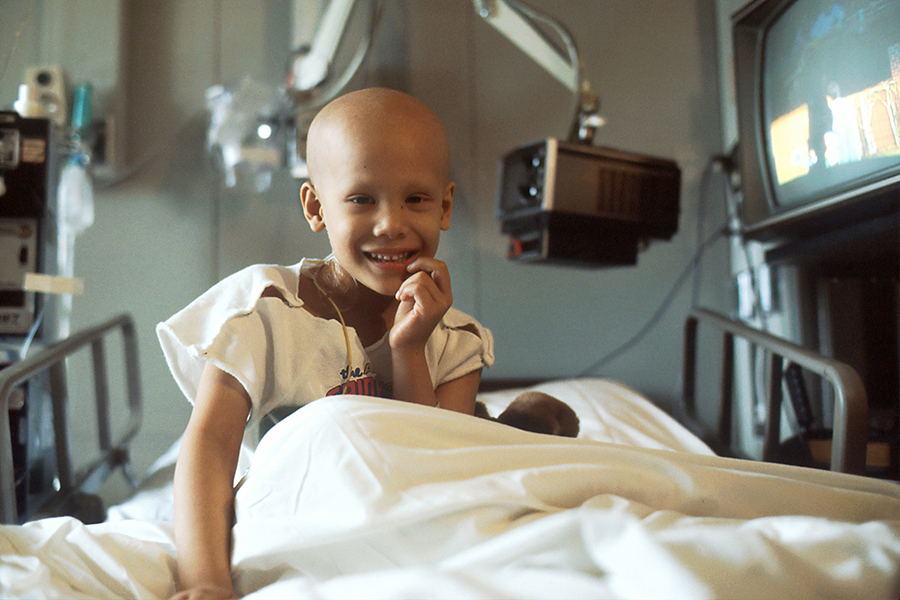
June 19 is a day of dual significance: World Sickle Cell Day and Juneteenth. It’s a day of celebration, acknowledging the remarkable progress in treating sickle cell disease (SCD). However, it’s also a day of reflection on the work still needed to address healthcare disparities.
SCD is a genetic disorder that causes red blood cells to become crescent-shaped, leading to severe health complications such as pain, fatigue, organ damage, and an increased risk of infections.
On World Sickle Cell Day 2023, we asked whether patients might soon have access to new treatments. On World Sickle Cell Day 2024, groundbreaking gene therapies are available.
A year of progress on sickle cell disease
In December 2023, the U.S. Food and Drug Administration (FDA) approved the first cell-based gene therapies for sickle cell disease: Casgevy by Vertex Pharmaceuticals and Lyfgenia by bluebird bio.
Casgevy (exa-cel) is the first FDA-approved medicine developed through CRISPR technology. The treatment involves taking a patient’s blood stem cells and modifying them with CRISPR-Cas9. The modified cells are transplanted back into the patient, where they attach and then multiply within the bone marrow. This increases the production of fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery. This therapy allows the levels of HbF to increase in patients and prevent the sickling of red blood cells in the future.
bluebird’s Lyfgenia, on the other hand, employs a previously used lentiviral vector (gene delivery vehicle) for genetic modification. This method takes a patient’s blood stem cells and genetically modifies them to produce HbAT87Q, a gene-therapy-derived hemoglobin that functions similarly to hemoglobin A (normal adult hemoglobin). These newly modified red blood cells have a lower risk of sickling and occluding blood flow. These modified stem cells are then delivered to the patient.
A new film, presented by Vertex Pharmaceuticals and produced for BIO by BBC StoryWorks Commercial Productions, tells the story of a patient and his daughter, who has the most severe form of SCD, and highlights the transformative impact of these new treatments:
Presented by Vertex Pharmaceuticals and produced for BIO by BBC StoryWorks Commercial Productions
The impact of SCD and historical inequities
SCD disproportionately affects people of African descent and has long been under-researched. Historically, Black Americans have faced significant disparities in medical research and treatment.
World Sickle Cell Day is the same day as Juneteenth, commemorating the end of slavery in the United States, also highlights the ongoing struggle for equity in healthcare, particularly for conditions like SCD.
SCD was first identified in the U.S. in 1910, but effective treatments were slow to develop. Blood transfusions became a treatment option in the 1960s, followed by stem-cell transplants in 1984. However, only a small fraction of patients had suitable donor matches. The 1990s saw the approval of hydroxyurea, the first medication for SCD, but comprehensive research remained minimal until recent advancements in gene-editing technologies.
This began to change in 2019 when the FDA approved Global Blood Therapeutics’ Oxbryta, the first drug to address the underlying cause of SCD. BIO Chair Dr. Ted W. Love, then the head of Global Blood Therapeutics, played a crucial role in this advancement.
“Just seeing how the patients had very few options, and quite frankly how they were treated in the hospitals, I felt that it was an indignity, and I felt it was really a moral imperative for someone to try to do something about it,” said Dr. Love, reflecting on his commitment to fighting the disease.
Moving forward with hope
As we celebrate World Sickle Cell Day 2024, we acknowledge the tremendous progress made in the past year. The approval of Casgevy and Lyfgenia offers new hope and a better quality of life for those living with SCD.
However, the journey is far from over. Continued research, equitable healthcare practices, and inclusive clinical trials are essential to ensure that everyone affected by SCD can benefit from these breakthroughs.
Header Image: 06/27/2023 – Boston, MA – Vertex Pharmaceuticals Learning Lab – Vertex Pharmaceuticals CEO Reshma Kewalramani visited with summer interns at Vertex’s Boston Learning Lab on June 27, 2023 on the second day of their internship. She addressed the larger group, and met individually with smaller cohorts to answer questions about science, careers, and her path to becoming the first woman of color to lead a large US biotech. Photo by Dina Rudick/Anthem Multimedia for Vertex Pharmaceuticals.