BIO Patient Advocacy Coffee Chat: Act now to be heard on CMS drug pricing
The Medicare drug price “negotiation” process has entered its third year, with some ...
Read More
Meet us in Miami for the BIO Investment and Growth Summit
The inaugural Biotechnology Innovation Organization (BIO) Investment and Growth (BIG) Summit kicks off ...
Read More
BIO warns MFN models are illegal, ineffective, and a threat to innovation
Proposals to let decisions by other countries control American drug prices are illegal, ...
Read More
Music Beats Cancer aims to bring everyone to the stage
The Valley of Death is a healthcare crisis, but the nonprofit, Music Beats ...
Read More
The BIG Summit meets at the intersection of patient advocacy and venture investment
Patient advocacy organizations that leverage venture philanthropy—an approach to philanthropic giving that applies ...
Read More
BIO Coffee Chat: MFN proposals would harm innovation and access
Most Favored Nation (MFN) type drug pricing proposals that were proposed by the ...
Read More
EDITORS' CHOICE
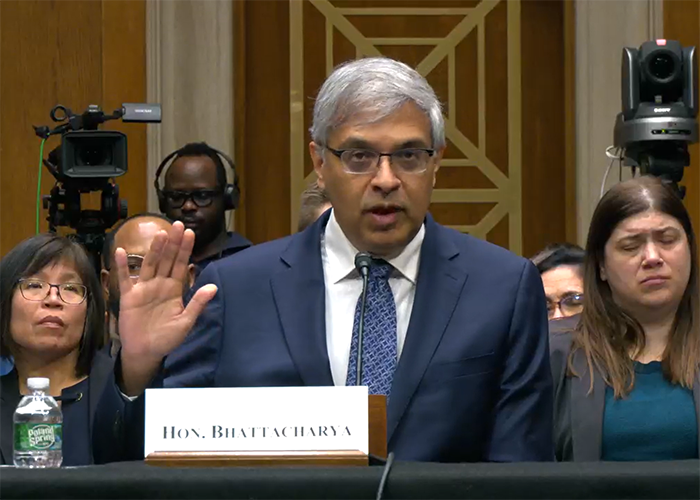
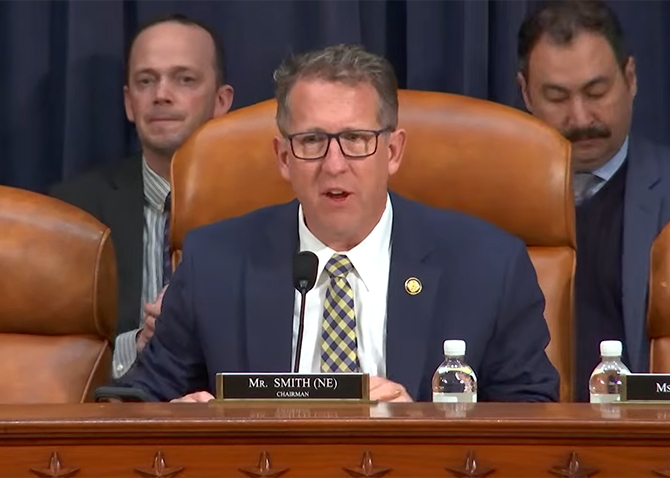
BIO CEO testifies in hearing highlighting PBMs’ impact on drug prices
Pharmacy benefit managers (PBMs) were identified as a major driver behind higher prescription drug costs in a congressional hearing that featured testimony from BIO President ...
February 13, 2026
Read More
LATEST NEWS
Federal Policy
BIO Patient Advocacy Coffee Chat: Act now to be heard on CMS drug pricing
The Medicare drug price “negotiation” process has entered its third year, with some evolution but continued complexity for patients and patient organizations. This year, 15 ...
February 26, 2026
Latest News
Meet us in Miami for the BIO Investment and Growth Summit
The inaugural Biotechnology Innovation Organization (BIO) Investment and Growth (BIG) Summit kicks off on March 2, 2025, and will convene industry leaders in sunny Miami ...
February 25, 2026
Latest News
BIO warns MFN models are illegal, ineffective, and a threat to innovation
Proposals to let decisions by other countries control American drug prices are illegal, would reduce the availability of innovative new treatments, and fail to help ...
February 25, 2026
Health
Music Beats Cancer aims to bring everyone to the stage
The Valley of Death is a healthcare crisis, but the nonprofit, Music Beats Cancer, has an innovative way to help solve it, and it starts ...
February 25, 2026
BIO Events
The BIG Summit meets at the intersection of patient advocacy and venture investment
Patient advocacy organizations that leverage venture philanthropy—an approach to philanthropic giving that applies venture capital principles to achieve philanthropic goals—can provide vital funding sources for ...
February 23, 2026
HEALTH
Music Beats Cancer aims to bring everyone to the stage
February 25, 2026
BIO Coffee Chat: MFN proposals would harm innovation and access
February 18, 2026
BIO CEO testifies in hearing highlighting PBMs’ impact on drug prices
February 13, 2026
BIO tells Congress how to maintain US biotech leadership
February 2, 2026
Welcome, John F. Crowley!
Get to know John F. Crowley, the new President and CEO of the Biotechnology Innovation Organization (BIO), in our exclusive interview.
Federal Policy
BIO Coffee Chat: MFN proposals would harm innovation and access
February 18, 2026
BIO CEO testifies in hearing highlighting PBMs’ impact on drug prices
February 13, 2026
BIO tells Congress how to maintain US biotech leadership
February 2, 2026
State Policy
Charlotte emerging as a global leader in health care innovation and med tech
December 10, 2025
Historically, Charlotte has been recognized as a major U.S. financial hub. Now, the city is making significant strides in another ...
Read More
BIO-CSBA report explains why and how states seek to attract biotech
December 2, 2025
BIO brings together patient advocates and industry at PACE
October 27, 2025
September Coffee Chat: The State of Patient Access
October 23, 2025
International
BIO 2025: The world can’t wait – it needs to collaborate
June 23, 2025
BIO IP expert reviews the current policy landscape
April 26, 2025
BIO’s mission to Tokyo builds on fruitful cooperation
March 25, 2025
USMCA dispute panel halts Mexico’s ban on biotech corn
January 6, 2025
Bio's View
BIO Investment Council will bring together VC and small biotechs to boost innovation
January 9, 2026
In an initiative to accelerate capital formation for biotech innovation, the Biotechnology Innovation Organization (BIO) on Jan. 7 launched the ...
Read More
BIO warns of risks from change to CDC’s vaccine recommendations
January 7, 2026
House passes BIO-backed legislation to increase access to capital
December 12, 2025
Oncologist warns PBM step therapy has ‘crossed a dangerous line’
November 20, 2025