The patchwork of state regulations regarding Medicaid coverage for new, innovative treatments can create barriers for patients seeking access to the most advanced therapies, including treatments for rare diseases, according to an analysis published by Avalere Monday.
Avalere’s study looks at 10 states and reviews “approaches to coverage and management for innovative therapies covered under the medical benefit” for Medicaid beneficiaries, who include children and the disabled. The research shows access to treatments can be inhibited by a lack of transparency or patient input, as well as other policy gaps. The report finds “opportunities to enhance consistency and transparency in the coverage decision-making process across state Medicaid programs.”
Medical benefit drugs are typically specialty drugs administered by health care providers instead of being given out by a pharmacy. While state coverage policies for pharmacy benefit drugs are similar, “processes for making coverage decisions for medical benefit drugs vary more significantly by state,” the report says. This category of drug includes many innovative cell and gene therapies, which offer major improvements in care but also face special regulatory barriers, as regulators seek to keep up with the science. Many treatments for rare diseases also fall within this category of drug.
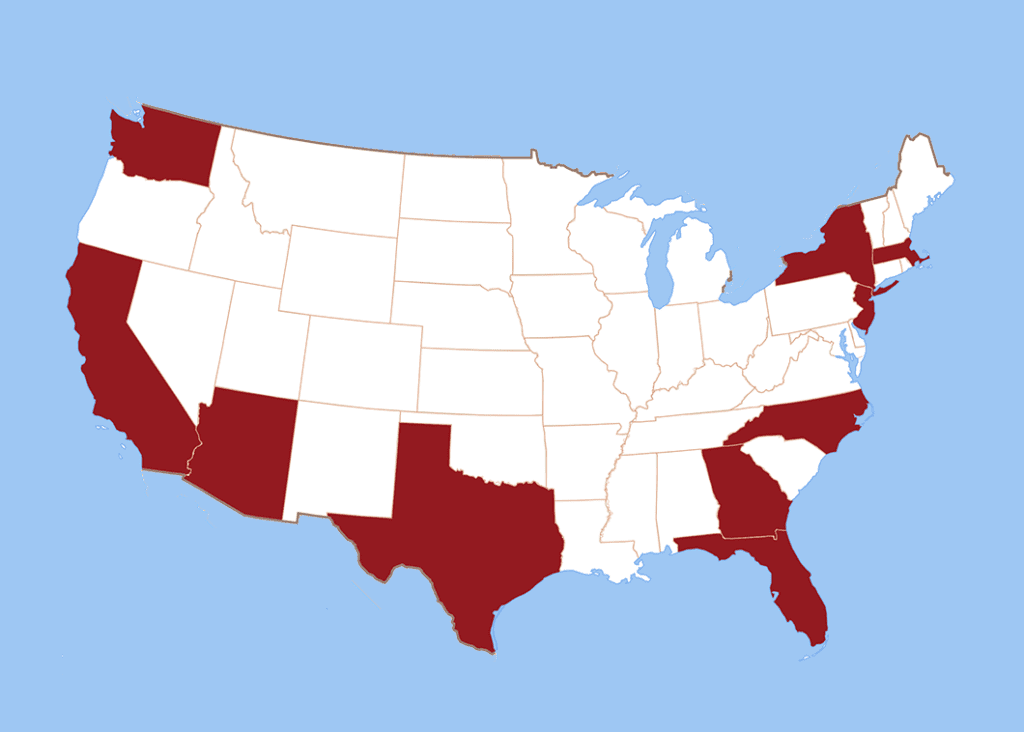
Avalere’s report reviews coverage and management decisions for two innovative medical benefit drugs in Arizona, California, Florida, Georgia, Massachusetts, New Jersey, New York, North Carolina, Texas and Washington.
“Currently, patient and caregiver experience and access to innovative therapies may vary considerably by state,” the report says.
The problem with a policy patchwork
According to the report, potential problems caused by diverse state policies that could limit patient access to new therapies include:
- In some states, there is limited sharing of public information, limited information on public meetings, and/or limited opportunities for the public to give comments. There are also limits on information provided by manufacturers in some states.
- Some states have not published their coverage policies on the drugs under review, and other states “had policies that were more restrictive than the FDA label for at least 1 of the innovative therapies.”
- Some states “Make coverage determinations on a case-by-case basis rather than implementing formal coverage policies.”
Given that 61.1% of enrollees in Medicaid identify as a minority the variations in state policy would seem likely to exacerbate health care disparities.
Avalere’s research goes into the differences in-depth. By identifying potential problematic policies, the report highlights “opportunities for increased consistency and transparency.”