On April 27, a group of bipartisan U.S. Senators and U.S. Representatives reintroduced the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act. The bill would support R&D for new antimicrobials and antibiotics.
“Right now, we don’t have the tools to address the threat posed by antimicrobial resistance – and infectious disease experts are warning us that it will only get worse,” said Sen. Michael Bennet (D-CO). “The bipartisan PASTEUR Act is the strongest bill ever written to strengthen antibiotic development and use. It will fix our market failures, expand the pipeline for next generation antibiotics, and save lives.”
What is the PASTEUR Act?
According to the Wall Street Journal, the bill would commit some $6 billion to “establish a committee of federal officials to decide in consultation with patients and doctors which new treatments approved by the Food and Drug Administration the federal government should purchase. Manufacturers could receive between $750 million and $3 billion for new drugs over several years.”
“Under this system, the government would enter into contracts with innovators to pay for consistent access to novel antimicrobials with payments that are decoupled from the volume of antimicrobials used,” explains Emily Wheeler, Director of Infectious Disease Policy at the Biotechnology Innovation Organization (BIO).
In addition to Sen. Bennet, co-sponsors include Sen. Todd Young (R-IN), as well as Reps. Scott Peters (D-CA), Drew Ferguson (R-GA), Mike Levin (D-CA), and Jake LaTurner (R-KS) in the House.
Why do we need the PASTEUR Act?
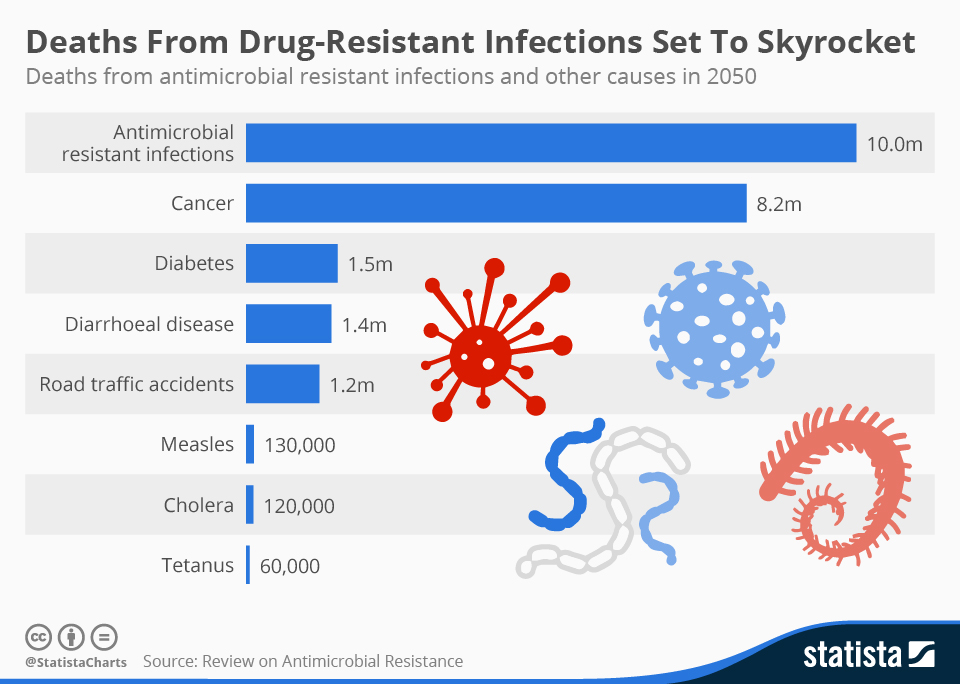
“Superbugs were linked to almost 5 million deaths worldwide in 2019, making AMR a leading cause of mortality worldwide,” wrote BIO’s Wheeler on Bio.News earlier this month.. “Unfortunately, the current state of research into antimicrobial treatments is nowhere near where we need it to be to effectively combat the AMR threats we face today, not to mention those that we will inevitably confront in the future.”
“For decades, we have seen AMR soar around the world, while the pipeline for new treatments slows to a trickle due to the broken ecosystem for antimicrobial innovation,” says BIO CEO Rachel King in a statement to Bio.News.
“The PASTEUR Act is an integral solution to addressing the global public health crisis of AMR. The bipartisan bill will help repair the foundational challenges of the antimicrobial marketplace and drive the development of new, innovative treatments for patients,” she continues.
“Drug-resistant infections can happen because of a scrape on a playground, the birth of a child, an organ transplant, or simply standing in line at the grocery store. The pervasive nature of multi-drug resistant superbugs, combined with the chronically broken pipeline of urgently needed new drugs, underscores the grim reality that we cannot wait any longer to pass the PASTEUR Act,” echoed a joint statement signed by BIO, the Cystic Fibrosis Foundation, the Infectious Diseases Society of America, the Partnership to Fight Infectious Disease, and the Pew Charitable Trusts.
Sens. Bennet and Young first introduced the PASTEUR Act in 2020.




