Pictured above, Rep. Deborah Ross (D-NC) addresses the opening session of BIO’s Intellectual Property Counsels Committee Conference in Washington, D.C., on Nov. 18.
The Biotechnology Innovation Organization (BIO) brought together top intellectual property experts to network and share insights on encouraging innovation by protecting ideas during BIO’s Intellectual Property Counsels Committee Conference in Washington, D.C., which took place November 18-20, 2024.
With IP being a linchpin of biotechnological innovation and the biopharma industry, BIO and its members are leaders in advancing and helping develop strong IP and patent protection.
Another leader in the field is Rep. Deborah Ross (D-NC), who has championed legislation to strengthen IP protection. She is a member of the Subcommittee on Courts, Intellectual Property, and the Internet.
Rep. Ross opened the conference with a keynote about the importance of strong, clearly defined, and enforceable IP rights. Rep. Ross, whose North Carolina district includes Research Triangle Park, also spoke of how the biotech sector has transformed the state of North Carolina to become an economic engine and a source of cutting-edge science and cures.
Also speaking was Judge Paluine Newman of the U.S. Court of Appeals for the Federal Circuit, appointed in 1984 by President Ronald Reagan. Spending most of her career as a key member of a specialized appellate court that hears all patent law appeals, Judge Newman is considered a leading figure in the patent and IP field and is greatly respected. Her address was warmly received, and she was given a standing ovation by those in attendance.
Highlights from BIO’s 2024 IP Counsels Committee Conference
A panel on “Empowering Patients and Driving Pharmaceutical Progress: A Multi-Stakeholder Dialogue” addressed the evolving role of patient advocacy groups and foundations during research and drug development, including grants, donations, and other research funding; equity positions in companies; working with patient communities on clinical trial design and recruitment; and patient representation on company advisory boards.
-

From left, Moderator Barbara Fiacco, Partner, Co-Chair of Intellectual Property Department, and Co-Chair of Patent, Trade Secrets, and Related Rights Litigation Practice”; Karin Hoelzer, Senior Director, Patient Advocacy, BIO; Javeed Froozan, VP, Business Development and Strategic Alliances – The Leukemia and Lymphoma Society, Inc.; Scott Carmer, CEO – T1D NewCo
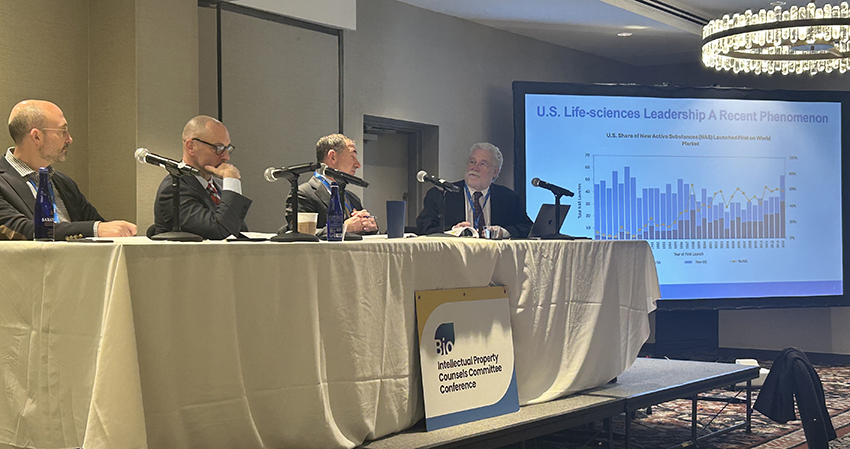
A panel entitled “What Comes Next? American Competitiveness in the Next Administration” looked at technology areas, approaches for fostering competitiveness, and the use of cooperation or confrontation to protect American interests at home and abroad.
-

From right to left: Moderator Kevin Noonan, Biotech and Pharma Practice Group Co-Chair – McDonnel Boehnen Hulbert & Berghoff LLP; Stephen Ezell, Vice President, Global Innovation Policy, and Director, Center for Life Sciences Innovation – Information Technology and Innovation Foundation (ITIF); Jeffrey E. Depp, JD, MSIA, PhD Candidate, Public & International Affairs – AUTM Public Policy Legal Task Force, Edison Fellow – Center for Intellectual Property x Innovation Policy (C-IP2) and Consultant – Center for Strategic and International Studies (CSIS); and Corey Salsberg, Vice President, Global Head IP Affairs – Novartis
Other panels explored the legislative landscape and prospects for action in the new Congress; strategies for biosimilars patent litigation; the evolving importance of patent opinions in defending against willful infringement claims; and developments in the European Union.