In 2022, Congress enacted the Food and Drug Omnibus Reform Act, also known as FDORA, which requires drug sponsors to submit diversity action plans to the U.S. Food and Drug Administration (FDA). A recent BlackDoctor.org Facebook Live panel discussion sponsored by the Biotechnology Innovation Organization (BIO) explored the progress since then, and what more needs to be achieved.
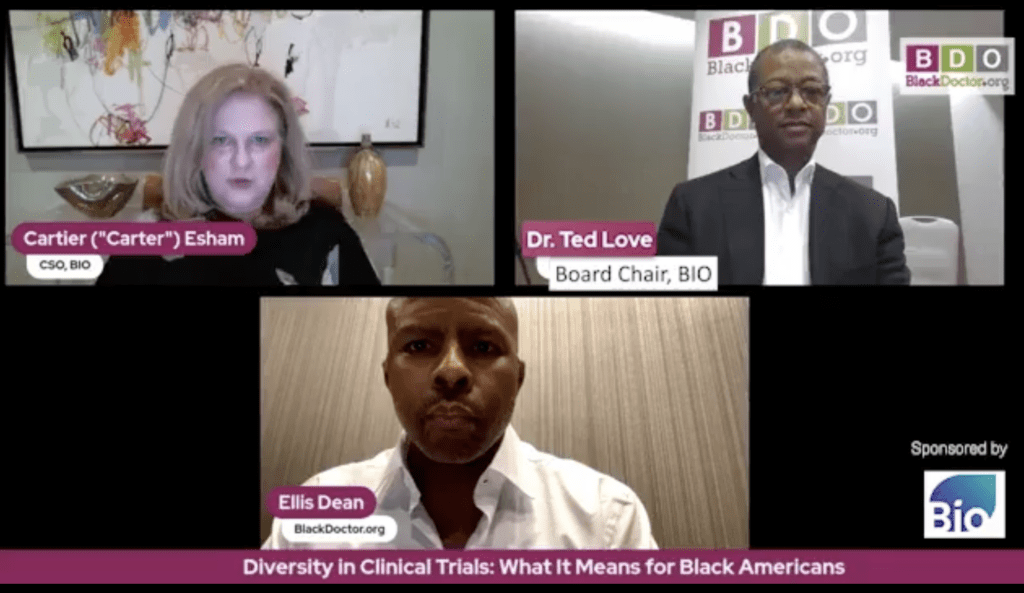
“We are losing a lot of opportunity by having clinical trials underrepresent certain groups,” said Dr. Ted W. Love, Chair of BIO and former President and CEO of Global Blood Therapeutics. “We don’t get the science from studying those populations—and sometimes the science could be different, and we don’t know until we study it.”
Four years ago, at the direction of BIO Board Members (including Dr. Love), BIO engaged on clinical trial diversity with the goal of not just looking at the problem, “but solving the problem,” said Dr. Carter Esham, BIO’s Chief Scientific Officer. “So, we got to work.”
BIO proposed a framework to the FDA—a lot of which was taken up by supportive Members of Congress—”to advance work and make clinical trials more representative,” she explained. This included “guidance and public dialogue about how to take learnings from what we saw in COVID,” as well as decentralized trials, to break down barriers to participation.
“All of those things will serve to better enable trials to be more representative of the patients that they’re treating,” continued Dr. Esham. “Now, we need to get work on implementation.”
In addition to leading to better, more inclusive science, there are practical reasons for improving clinical trial diversity.
“By having a broader patient population, you will enroll in your clinical trials more quickly,” said Dr. Love. It also facilitates the uptake of the therapy.
“There are lots of reasons for companies to do this,” noted Dr. Love.
Why do clinical trials lack diversity?
“It starts, in part, with the bias. There are many physicians who have a negative view of even recruiting people of color in their clinical trials,” said Dr. Love.
In other words, patients aren’t even asked.
Many patients in diverse communities are in jobs where they can’t simply take off work in the middle of the day—and he saw this firsthand at Global Blood Therapeutics, where patients with sickle cell disease said they already had a hard enough time keeping a job that they couldn’t justify voluntarily missing work to participate in a trial. This is why a key step toward increasing clinical trial diversity is by adding greater adaptability and flexibility in the trial processes—such as by providing stipends or opening trial sites off-hours, he explained.
The biopharmaceutical sector is “a very conservative business,” he explained. “We tend to do things the same way over and over and over again.” However, he’s confident FDORA will “stimulate people to change how they do things.”
“I think they’ll be happy with the outcomes they get,” he said.
How to improve clinical trial diversity, step by step
Earlier in the discussion, Dr. Esham explained that she’s been working in biotechnology for 15 years: “15 years ago, we weren’t able to say that we provide cures—and now we can. That’s a pretty amazing transition.”
Now, it’s important to ensure those cures are inclusive and accessible, from the very start of the clinical trial process. But building trust within communities of color is key to getting to more diverse trial populations.
Explaining the reasons why the Black community, in particular, doesn’t participate, “We hear Henrietta Lacks. We hear Tuskegee. We hear, my mama had a problem,” said BlackDoctor.org’s Ellis Dean. “What are some things we can possibility do to help chip away some of that historical distrust and mistrust?”
“Honestly, this is a solution. As long as clinical trials continue to be a hypothetical in people of color’s communities, we’re never going to get the true facts,” replied Dr. Love. “We’re going to break down that through education and knowledge. And that’s going to come about through engagement in clinical studies.”
BIO has prioritized this, working with the FDA, member companies, and partner organizations (like BlackDoctor.org) to rethink clinical trial protocols, development, and review processes, convene important conversations about best practices or policy development, and promote diverse hiring within biotech companies, explained Dr. Esham.
BIO also developed a “patient-friendly website,” CTPOP.org, to empower patients and their families to identify and evaluate clinical trial opportunities.
Where will this lead—and what will clinical trials look like five years from now?
“I expect clinical trials to be more representative of the patients they are treating than they are today,” said Dr. Esham. However: “I do not think we’ll be done,” she added, noting the importance of continued engagement with communities, physicians, and companies.
“The arc of time bends towards justice, but it’s very long,” added Dr. Love, paraphrasing Dr. Martin Luther King, Jr. But one day, clinical trials will be truly representative of the patient communities they serve.