Exciting new therapies promise to transform medicine. But ensuring patients can access these complex treatments may require innovative payment models, says a study published May 4.
Transformative therapies, which include cell and gene therapies, offer new treatment options for patients, often addressing conditions for which there are currently limited or no treatment options available. They are durable, so one treatment could last several years, or even a lifetime.
New transformative therapies that are currently in development also may require intensive patient treatment. And these therapies may not fit neatly into current payment systems, according to the report, Pipeline Transformative Therapies, published by health care research firm Avalere. The issue has become more important because of the rapid increase in such treatments, either under development or already approved by the U.S. Food and Drug Administration (FDA).
“Due to the unique characteristics of transformative therapies (e.g., potentially durable nature, novel mechanisms of action, up-front treatment costs) stakeholders have raised the need for alternative payment and financing approaches that better account for these characteristics by recognizing both payer financing pressures and clinical value of the products,” the study says.
A big pipeline of transformative therapies
The study analyzes the impact of 214 transformative therapies currently under development. The goal is to assess the impact of these therapies on payers, providers, and patients.
Avalere focused on product/therapy combinations that met the “transformative therapy” criteria. Among the 214, one-third are meant to treat cancer. Therapies treating neurologic, metabolic, ophthalmologic, and autoimmune conditions each account for an additional 10% or more of therapies in development.
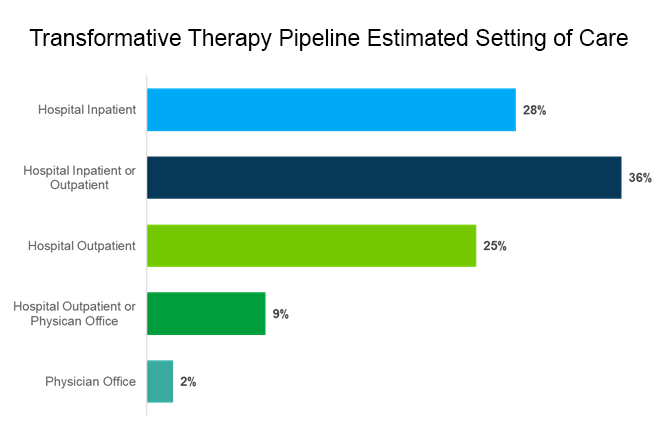
Of these therapies, 82% would require only one application. In addition, 89% are intended to be administered in a hospital, either in an inpatient or outpatient setting.
Why transformative therapies need a different payment model
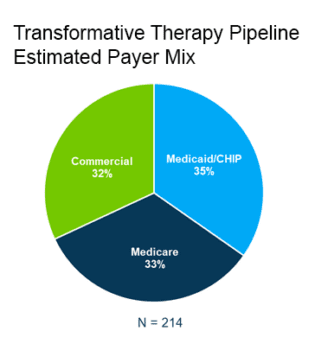
Avalere estimates government-sponsored insurance will cover two-thirds of the transformative therapies, while Medicaid/CHIP will be the primary payer for 35%.
This diverges from insurance coverage of all services in the U.S. population. More than 50% in the U.S. are covered by commercial insurance and 20% are covered by Medicare/Medicaid.
These new treatments require different payment models, according to the report.
“Traditional payment models, such as the bundled payments used under Medicare Fee-for-Service (FFS) in the inpatient setting, were not designed to accommodate many of the particular characteristics of transformative therapies,” the report says.
The U.S. Department of Health and Human Services in February instructed the Center for Medicare and Medicaid Innovation (CMMI) to advance a payment model for ensuring patient access to cell and gene therapy. The proposed response requires more detail, according to Avalere.
The Biotechnology Innovation Organization (BIO), a sponsor of the report, noted the need for faster reform. While innovation keeps occurring in the lab, we also need innovation in payment mechanisms to bring the growing set of transformative therapies to the patients who need them, according to BIO.