People six months and older should have one of the updated COVID-19 vaccines released by Pfizer-BioNTech and Moderna, under the recommendation approved Sept. 12 by the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP).
The recommendation comes the day after the Food and Drug Administration (FDA) approved the updated vaccines for people over 12 and gave emergency use authorization for children ages 6-11, as Bio.News reported.
“Everyone 6 years and older should get 1 updated Pfizer-BioNTech or Moderna COVID-19 vaccine, regardless of whether they’ve received any original COVID-19 vaccines,” according to the CDC’s Sept. 12 recommendation, which was approved by CDC Director Mandy Cohen. For those 6 months to 5 years old, 2-3 doses total, including one updated vaccine, are recommended.
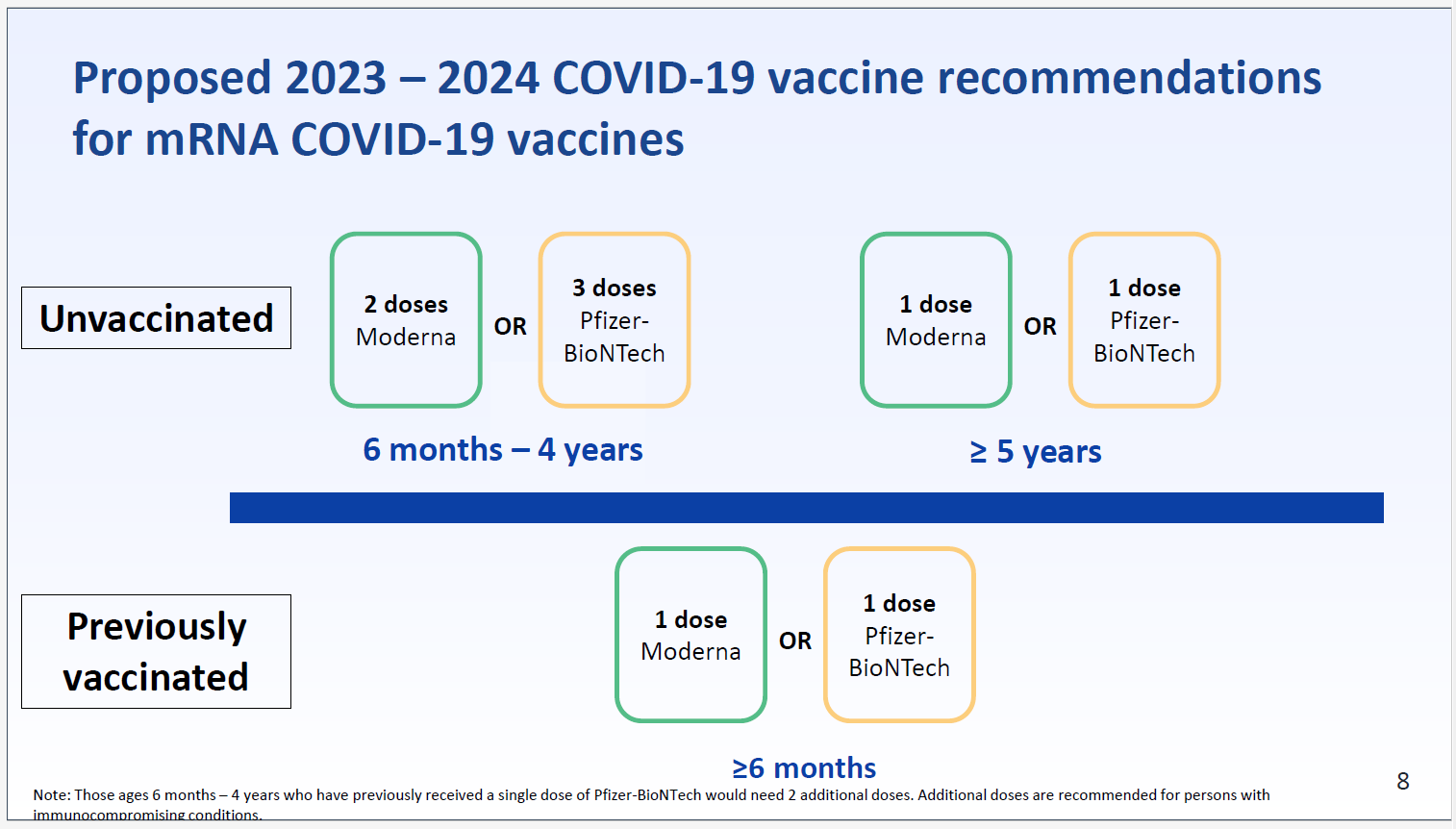
The Pfizer-BioNTech and Moderna mRNA vaccines are updated to target the Omicron XBB.1.5 mutation of the virus, per the request in January from FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC). Both shots also have proven effective against the now-dominant EG.5 (Eris) variant and newer BA.2.86 (Pirola) variant.
Another updated vaccine, made by Novavax using protein-based technology, awaits FDA approval.
The passage of the ACIP’s recommendations came as COVID-19 posed a heavier burden over the end of summer season. “Hospitalizations have jumped more than 21% compared to the prior week,” reported NPR in late August. “Some hospitals and schools have reinstated mask requirements or at least actively encouraged people to wear them again.”
Pfizer and Moderna said the new shots will be available this week. They are given in single doses, instead of the two shots used in previous vaccines.
The ACIP meeting also featured important presentations on the latest research about COVID-19, all of which can be found on the ACIP website.
Importance of vaccines for children
According to one presentation, the highest percentages of hospitalizations for COVID-19 were among children who were not vaccinated, or whose vaccinations had been allowed to expire, a fact that supports regular vaccination.
Among hospitalized children who had contracted COVID-19, ages 5–17, from January to June 2023, the CDC found that underlying conditions, especially among the very young, were not an indicator of whether a child would need hospitalization or not—it’s vaccination that matters.
Additional CDC findings for COVID in the United States include:
- Hospitalization rates increased in all age groups since mid-July;
- Hospitalization rates are highest in older adults and infants <6 months;
- COVID-19 continues to cause severe illness; clinical outcomes are generally comparable to influenza-associated hospitalizations; and
- Most children and adults hospitalized for COVID-19 since January 2023 had not received an updated bivalent booster.
The risk of repeated COVID-19 infections
There was also discussion of long COVID and the risks of repeated COVID-19 infections.
“There is literature and evidence that damage accumulated from SARS-CoV-2 infections is cumulative with each infection. So, although many people have been exposed to viral antigens through vaccination, infection or both, you can see physical damage being cumulative in the population. And increased mortality six months out has also been demonstrated in the literature,” said Dr. Melanie Mathew.
Notably, the issue of long COVID has been an ongoing concern among patients, especially as repeat contractions threaten to exacerbate the risk, with almost 1 in 4 adults with long COVID reporting significant activity limitations.
One study group presenting to ACIP found that “A wide range of physical and mental health consequences continue or develop at least 4 weeks after initial COVID-19 or SARS-CoV-2 infection.” The general consequences of this include Post-ICU syndrome and other complications of treatment or illness.
The prevalence of ongoing symptoms lasting at least three months after COVID-19 was highest among people aged 35–49 years, followed by those aged 50–64, and 18–34 respectively. Additionally, people of the female sex were at a greater risk of developing long COVID, as well as those of lower socio-economic status, and those who were unvaccinated.
However, the prevalence of long-COVID decreased from June 2022 to January 2023, while remaining unchanged through June 2023.
The study group also found that “following acute-COVID illness among adults, on-going symptoms decreased after 3 months, but 16% continued to experience on-going symptoms at 12 months.” Additionally, “Many adults reported new emerging or re-emerging symptoms at 6, 9, and 12 months following acute COVID-like illness.”
Instances of long COVID not only increase the U.S. healthcare burden but personal and social economic burden as well. Showing that the path forward for the fall is clear: get vaccinated.
Vaccine confidence needs a boost
The meeting also covered vaccine confidence and access, which differ among varying socio-economic ranges and ethnicities, according to a presentation. While there was “no evidence to suggest that COVID-19 vaccine effectiveness varies substantially by race/ethnicity,” there are “differences in vaccine hesitancy/uptake, crowding, access to care, and prior infection [that] that could impact vaccine effectiveness and these factors may also differ by race/ethnicity.”
This is why programs like the CDC’s Bridge Access Program, which aims to provide vaccine access for uninsured Americans (through December 31, 2024), are so important. However, more important is the addition of the Vaccines for Adults program, which is part of the President’s upcoming budget proposal, and which was referenced repeatedly by presenters of the Bridge Access Program.
Overall, the innovation associated with vaccines has changed the landscape of American healthcare. The only step now is for patients and doctors to make sure everyone gets the shots they need next week as the new monovalent vaccines become available.