When it comes to long COVID, health care providers and the biotech industry must be aware of what is already known, but we still need to learn much more, participants at an online long COVID webinar co-organized by the Biotechnology Innovation Organization (BIO) were told yesterday.
“While significant research efforts by the U.S. and other governments’ academic institutions and health care organizations are currently underway, private sector involvement and regulatory clarity are crucial for timely success,” Cartier Esham, Chief Scientific Officer for BIO, said in introducing the event “Long COVID: What will it take to accelerate therapeutic progress.”
For the next three hours, presenters at the webinar, the first gathering in a series co-organized with Solve M.E., provided the kind of information that the biotech community and other stakeholders need to tackle the challenge of long COVID.
Participants were told the condition has similarities to other post-infection disorders that have been studied for a long time, such as chronic fatigue syndrome. “I believe we can leverage the existing science and the commonalities experienced by patients with all these conditions,” said Dr. Rachel Levine, an admiral and assistant secretary for health at the U.S. Public Health Service.
They also learned of legislative progress and the work that needs to be done from Sen. Tim Kaine (D-Va.), who is a long COVID sufferer himself.
And they heard of the economic cost, put at $27-54 billion and growing in lost wages alone, from Katie Bach of the Brookings Institution, who said the millions of long COVID sufferers who could not work were helping drive the recent labor shortage.
Defining the challenge
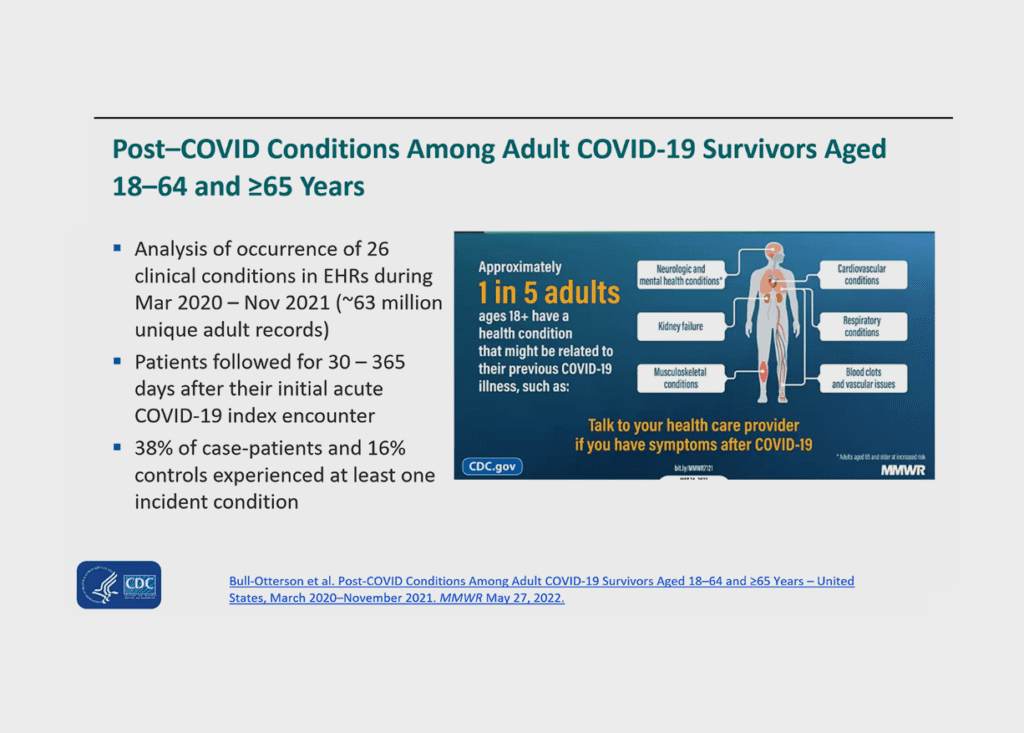
Dr. Priti Patel, a medical officer with the Centers for Disease Control and Prevention (CDC), said the CDC talks about “post-COVID conditions,” not just long COVID, to avoid an overly narrow definition. She outlined a range of neurological, cardiovascular, digestive, and other symptoms that have been observed. She said there is still no clear count of people with post COVID conditions, but 14.6% of adults who have had COVID report having long COVID.
When it comes to defining long COVID, it was appropriate to hear from a patient representative because as she, and other presenters noted, it was patients who first identified the condition and came up with the term “long COVID.” Lisa McCorkell of the Patient-Led Research Collaborative gave a history of how the patient-led movement encouraged research and described the challenges of understanding long COVID, which manifests in many different ways and impacts an average of nine different organs in the human body.
McCorkell also described the challenges patient advocates face. “In order to get better, we must spend energy advocating for ourselves, but in order to not get worse, we must rest.” She said patient advocates had many desires, but a key one was to see more clinical trials—which should not be limited to those who have had a positive COVID test.
Dr. David Putrino of Mt. Sinai Hospital in New York agreed that anyone who may have been exposed to COVID, whether or not they tested positive for the virus, could have long COVID. He noted the CDC and WHO have said that, given the many potential symptoms of long COVID and the lack of full understanding, a doctor’s clinical diagnosis should be used, as there are no conclusive diagnostic tests.
“We don’t need testing, we don’t need biomarkers, we just need to listen to our patients,” he said, adding that payers cannot demand lab tests to confirm a doctor’s diagnosis. “Please let us stop asking sick people to prove that they’re sick.”
The search for solutions
The information became more scientific and specific as Dr. Akiko Iwasaki of Yale presented research based on the various hypotheses for root causes of long COVID that have been studied, including looking at viral reservoirs, autoimmunity, reactivation of a latent virus, and tissue damaged from inflammation.
Dr. Walter Koroshetz from the NIH’s National Institute of Neurological Disorders presented important findings achieved thus far by his agency’s four-year, $1.5 billion RECOVER study.
Presentations from biotech companies that are preparing or conducting clinical trials with drug candidates, including candidates that have shown promise against other post-infection diseases, represented AIM ImmunoTech, Axcella, and IncellDx.
The conversation and quest for a long COVID solution continues in Boston this June, as part of the BIO International Convention, which runs June 5-8.