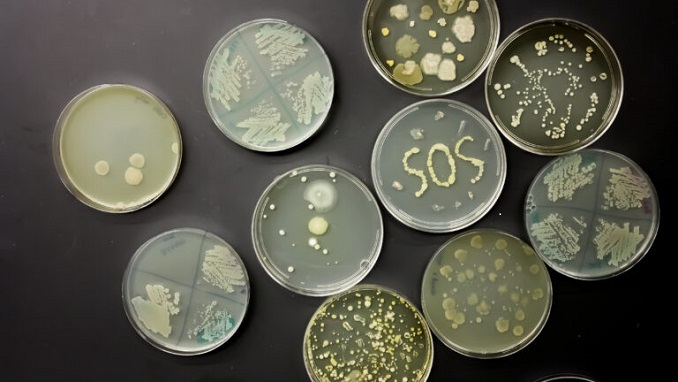
“The COVID-19 pandemic pushed back years of progress made combating antimicrobial resistance (AMR) in the United States,” the Centers for Disease Control and Prevention (CDC) warned in its report published last week. The report underscored that “antimicrobial-resistant infections and deaths increased 15% in hospitals in 2020.”
CDC started painting the grim image of AMR back in 2019 in its Antibiotic Resistance Threats in the United States report, noting that “more than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and at least 35,000 people die as a result of these superbugs.”
Antimicrobial resistance (AMR) is already one of the most pressing health challenges of our time. This is why we have to act now to respond, two U.S. Senators and Biotechnology Innovation Organization (BIO) President and CEO Dr. Michelle McMurry-Heath agreed during the virtual event organized by Pfizer and CQ Roll Call on Wednesday, as reported by Good Day BIO.
“AMR superbugs are expected to kill more than 10 million people annually by 2050,” BIO’s Dr. Michelle McMurry-Heath noted, emphasizing the lack of “an arsenal of antimicrobials to fight resistant infection.”
“The time to develop those new drugs is now, but there are only 64 new antibacterial drug programs in development worldwide,” she underscored.
Pointing toward “our flawed antimicrobial market,” Dr. McMurry-Heath explained that “drug manufacturers need stronger financial incentives to build the drug pipeline of much-needed, life-saving measures.”
As we reported earlier this year, “funding for R&D of these drugs has declined, as this class of medicines does not yield blockbuster sales.” As panelists also noted, “companies developing antimicrobials often go broke before—or immediately after—they reach the market.”
“Right now, our system rewards volume, not value,” said Sen. Michael Bennet (D-CO). We need a pandemic-style “strategic partnership between biotech and the federal government, which is how we broke every record for vaccine development.”
PASTEUR Act to combat antimicrobial resistance has bipartisan support
The PASTEUR Act, sponsored by Rep. Mike Doyle (D-PA) and Drew Ferguson, DDS, (R-GA), reached 60 co-sponsors this week. The bill promotes the idea of public-private partnerships to combat the threat of new AMR infections. This signals the increased support to urgently tackle AMR, notes Good Day BIO.
Back in March, Rep. Ferguson called for investing in AMR pandemic preparedness “before we reach the breaking point where we can no longer defeat infections.” In the op-ed for The Washington Times, he underscored that the PASTEUR Act “would stabilize this market, incentivize the development of new drugs targeting the most threatening infections, improve the appropriate use of antibiotics, and ensure domestic availability when needed.”
This important legislation co-sponsored by Sen. Bennet and Sen. Todd Young (R-IN) “also provides the American taxpayer a return on the financial investments the U.S. makes in new antibiotic development.” The bill “would create a subscription-style model” whereby the government pays drug developers “based on a treatment’s value to public health.”