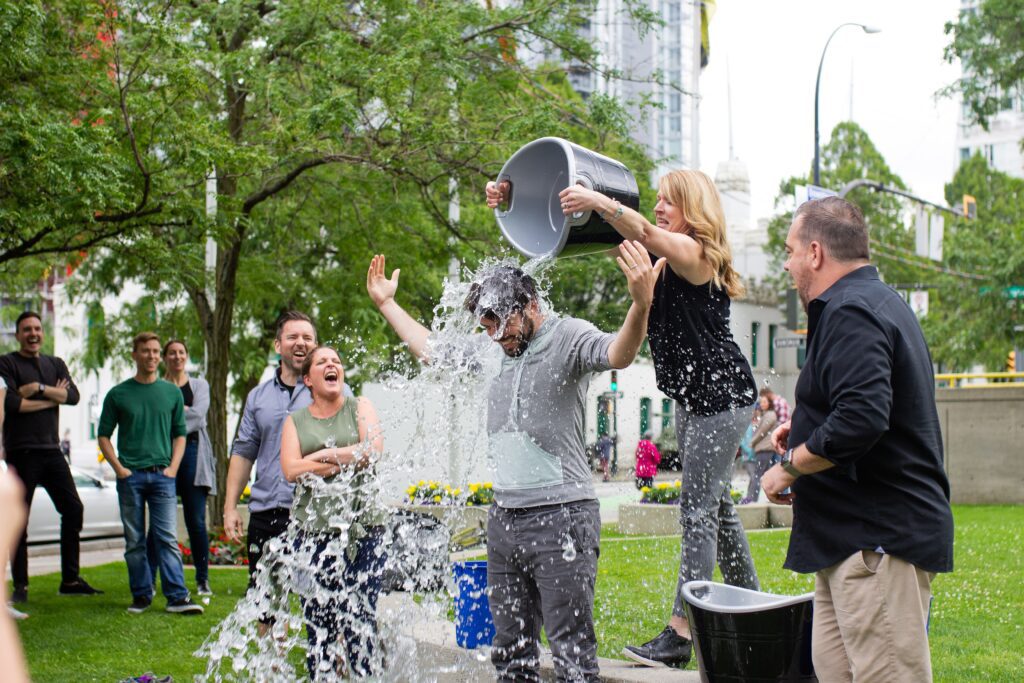The Food and Drug Administration (FDA) on Thursday authorized a new medication intended to halt the progression of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, the agency announced via press release.
“This approval provides another important treatment option for ALS, a life-threatening disease that currently has no cure,” said Billy Dunn, M.D., director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research. “The FDA remains committed to facilitating the development of additional ALS treatments.”
The ALS drug is produced by the Massachusetts-based pharma company Amylyx Pharmaceuticals and combines “sodium phenylbutyrate, which is prescribed to treat a metabolic disorder, and taurursodiol, an over-the-counter supplement used to help prevent liver disease,” NBC News explains.
What is ALS?
ALS is a neurodegenerative condition that causes motor neuron loss in the brain and spinal cord. ALS-related motor neuron loss causes declining muscular function, inability to move and talk, respiratory paralysis, and, subsequently, death.
More than 90% of persons with ALS have no apparent family history. ALS affects around 29,000 people in the United States.
“The published data on both function and survival in a randomized trial – and what this means for people living with ALS – are a step forward for the ALS community,” said Sabrina Paganoni, M.D., Ph.D., principal investigator of the CENTAUR trial.
What we know about the new ALS drug
The drug was developed partly with funding from the “Ice Bucket Challenge,” the viral social media campaign in which participants dumped buckets of ice water over their heads to raise awareness and money for the ALS Association.
The campaign started in 2014, eight years before the drug was approved. It’s the first new ALS drug approved in five years.
[embedyt] https://www.youtube.com/watch?v=qgqsgXSJ7g8[/embedyt]
“In 2014, the organization raised $115 million in six weeks from the Ice Bucket Challenge and provided $2.2 million of that to help pay for testing AMX0035, the drug’s name during development,” reports The Washington Post. “The medication is the first funded by the organization to receive FDA approval. Amylyx has agreed to use proceeds from sales of the medication to repay the organization 150 percent of its investment.”
There has been some controversy surrounding the drug. According to reports, when the medicine appeared before an FDA advisory group for the first time in March, advisers raised questions about the study data. The FDA questioned the Amylyx trial’s persuasiveness in briefing materials made public before the vote. By a slim margin, the committee decided against recommending approval.
Just six months later, when Amylyx had submitted a second analysis of the trial results, the FDA convened a second advisory committee, which then decided to recommend the drug, NBC News explains.