Biopharmaceutical company Global Blood Therapeutics, Inc., a member of the Biotechnology Innovation Organization (BIO), announced Monday that two of its treatments undergoing clinical trials for sickle cell disease (SCD), inclacumab and GBT021601 (GBT601), were granted both orphan drug and rare pediatric disease designations by the U.S. Food and Drug Administration (FDA).
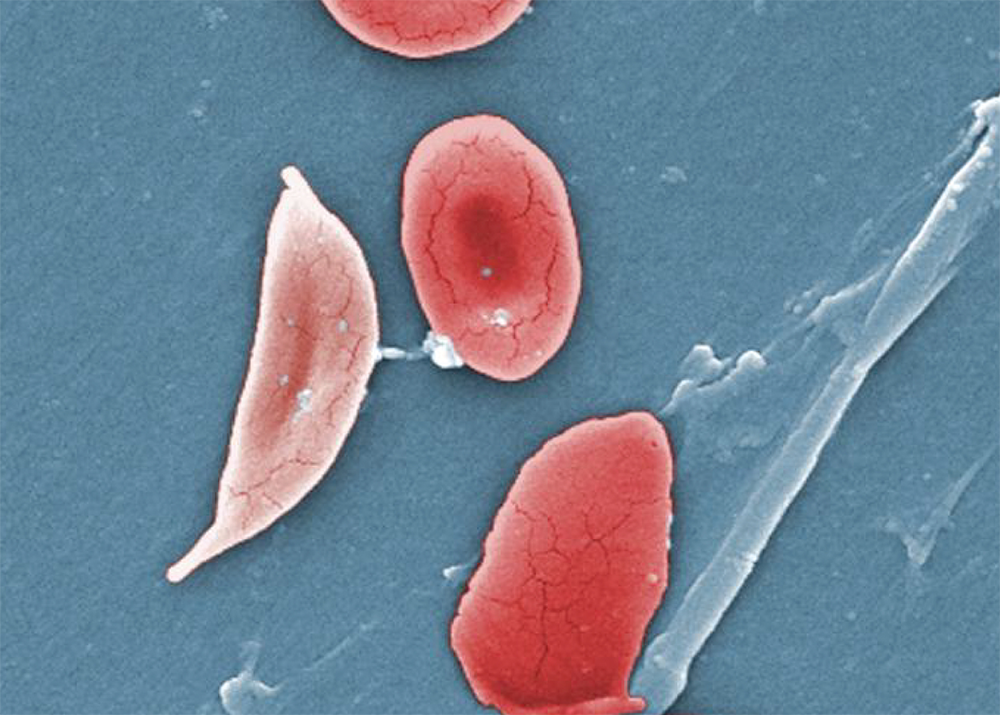
As Good Day BIO explained: “Sickle cell disease is an inherited red blood cell disease that can cause debilitating pain and lifelong complications. In the United States, the disease disproportionately affects African Americans.” The disease impacts hemoglobin, a protein carried by red blood cells that deliver oxygen to tissues and organs throughout the body.
“Inclacumab is a novel, fully human monoclonal antibody that selectively targets P-selectin, a protein that mediates cell adhesion and is clinically validated to reduce pain due to VOCs (vaso-occlusive crises) in people with SCD,” the company said. “Preclinical results suggest that inclacumab has the potential to be a best-in-class option for reducing VOCs in people with SCD, with the potential for quarterly, rather than monthly dosing.”
GBT601, which was developed by GBT’s research team, “is a next-generation sickle hemoglobin (HbS) polymerization inhibitor,” according to the company’s press release. The new treatment has the same mechanism of action as another drug developed by GBT, Oxbryta® (voxelotor). Approved by the FDA in November 2019, “Oxbryta is an inhibitor of deoxygenated sickle hemoglobin polymerization, which is the central abnormality in sickle cell disease,” according to the FDA.
Compared to the first-generation Oxbryta, GBT601 has “the potential for greater efficacy by achieving higher hemoglobin levels and occupancy at lower doses,” the company said. GBT601 “achieved a high target hemoglobin occupancy at daily doses lower than 500 mg, while maintaining a favorable safety and tolerability profile.”
Inclacumab is currently in Phase 3 clinical trials to evaluate its potential to reduce the occurrence of VOCs and readmissions due to VOCs, and GBT601 is being studied in a restarted Phase 1 clinical trial and is expected to advance into the Phase 2 portion of a Phase 2/3 trial by mid-year, the company said.
Taken together, the FDA orphan drug and rare pediatric disease designations, which were granted for both inclacumab and GBT601, provide market incentives for development and can facilitate priority review.
“The FDA’s orphan drug and rare pediatric disease designations for both inclacumab and GBT601 are an acknowledgment of the critical and ongoing unmet need in sickle cell disease and the potential of GBT’s innovative pipeline of investigational medicines,” said Kim Smith-Whitley, M.D., executive VP and head of research and development at GBT. “We believe that both inclacumab and GBT601 have the potential to be best-in-class therapeutic options for the treatment of this devasting disease. We are excited to continue these clinical development programs to make progress on our goal of transforming sickle cell disease into a well-managed condition via multiple therapeutic approaches.”