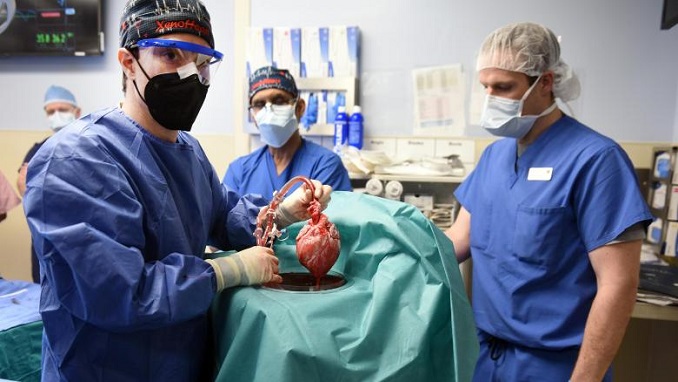
When a team of surgeons at the University of Maryland Medical Center successfully transplanted a pig’s heart into human for the first time ever, they not only gave a 57-year-old with a terminal heart condition a second chance, but they also paved the way for organ transplants that involve xenotransplantation and gene editing.
“This was a breakthrough surgery and brings us one step closer to solving the organ shortage crisis. There are simply not enough donor human hearts available to meet the long list of potential recipients,” said Dr. Bartley P. Griffith, who performed the transplant.
Xenotransplantation, the process of grafting or transplanting organs or tissues between members of different species, is propelled by demand for human organs for clinical transplantation that far exceeds the supply. The procedure has become more feasible thanks to advances in gene editing.
How xenotransplantation can offer hope to patients
The Food and Drug Administration (FDA) defines xenotransplantation as any procedure that “involves the transplantation, implantation or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues or organs that have had ex-vivo contact with live nonhuman animal cells, tissues or organs.”
According to UNOS, the nonprofit United Network for Organ Sharing that coordinates organ procurement efforts in the United States, a record 41,354 Americans received an organ transplant last year. But that’s not even half of the more than 106,000 people on the U.S. transplant waiting list.
Xenotransplantation offers hope to the hundreds of thousands of patients with failing organs who are waiting for a donor organ.
Although the potential benefits are considerable, there are also considerable risks from the procedure, first and foremost due to the higher risk of transplant rejection.
Science reports that “the problem is that antibodies produced by people recognize certain sugars on the surface of pig cells as foreign.”
How gene editing makes organs compatible
The response to this challenge has come from the science of gene editing. By editing the donor’s genes, scientists can make a non-human organ more viable for transplant in a human being.
The so-called Xenoheart used in the procedure, as Good Day BIO reports, came from a pig that received alterations to 10 genes by scientists at Virginia-based Revivicor, a subsidiary of United Therapeutics. The company is a member of the Biotechnology Innovation Organization (BIO).
The genetically modified donor pig used in the procedure was a GalSafe pig, from Revivicor.
GalSafe pigs are domestic pigs exposed to intentional genomic alteration (IGA) that edits their genes so they do not produce sugar that triggers organ rejection as well as an increasingly common meat allergy, according to the FDA. The FDA approved gene editing to grow GalSafe pigs in 2020, and gave emergency use authorization for use in a transplant in December, to allow this life-saving operation to take place.
Four of this pig’s genes were inactivated on top of the six human genes that were inserted into its genome, the New York Times explains. One of the alterations was made by removing the gene for a growth hormone receptor to stop excessive growth of the implanted pig tissue, while other alterations were aimed at reducing the chances the patient’s body would reject the new heart post-transplant.
A new immunosuppressant
Another innovation that made the historic operation possible was an immunosuppressant, developed in collaboration with Dr. Muhammad Mohiuddin, Director of the University of Maryland School of Medicine’s cardiac xenotransplantation program and produced by Kiniksa Pharmaceuticals. Their experimental antibody drug, KPL-404, is designed to prevent the body from immediately fighting back against the foreign implant.
According to Science, KPL-404 shuts down the excessive production of antibodies by the immune system against the organ, “by binding to a cellular receptor called CD40, suppressing the activity of antibody-producing B cells, and inhibiting their cross-talk with T cells that coordinate the immune system’s response to an invader.”