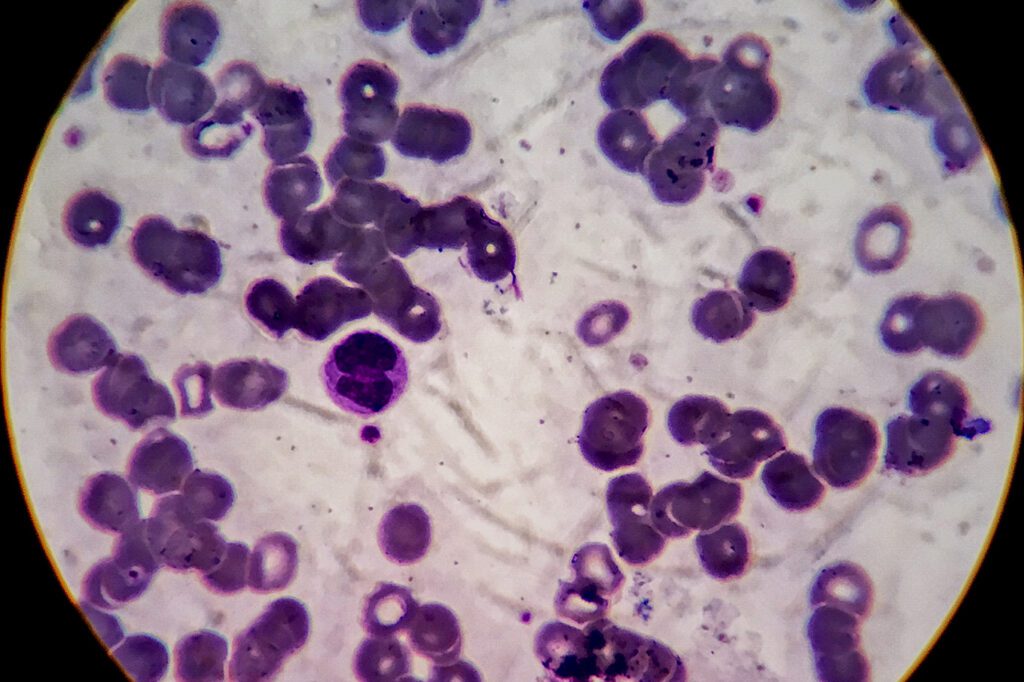
An innovative cell-based gene therapy implemented almost a decade ago not only eradicated cancer in a pair of chronic lymphocytic leukemia patients, it also provided researchers with “surprising findings of the long-term potential and clonal stability of the infused cells,” according to new research published in Nature magazine.
Genetically engineered CAR T cells stayed in the patients’ bloodstreams, providing continued immunity, even though there was no sign of leukemia for the cells to attack, the research said.
“Our identification and characterization of these unexpected CAR T cell populations provide novel insight into the CAR T cell characteristics associated with anti-cancer response and long-term remission in leukaemia,” the researchers involved wrote in Nature. “CAR T cells remained detectable more than ten years after infusion, with sustained remission in both patients.”
“Now we can finally say the word ‘cure’ with CAR T cells,” Dr. Carl June, one of the principal researchers in the study said, according to The New York Times, which quoted another researcher, Dr. David Porter, as saying: “Oncologists don’t use words like ‘cure’ lightly or easily or, frankly, very often.”
Growth in CAR T cell therapies
“In CAR T cell therapies, T cells are taken from the patient’s blood and are changed in the lab by adding a gene for a man-made receptor (called a chimeric antigen receptor or CAR). This helps them better identify specific cancer cell antigens. The CAR T cells are then given back to the patient,” according to the American Cancer Society.
Such so-called “living drugs” are now used by thousands around the world to treat certain blood cancers, according to the Associated Press (AP). The treatments reportedly work for some people but not others, and scientists are still trying to determine why.
This Associated Press video explains CAR T cell therapy:
The treatment, which has already received Food and Drug Administration (FDA) approval, is only available to patients in which at least two other types of standard treatment have failed.
Meanwhile, FDA has approved four more CAR T cell therapies to treat B-cell lymphoma—the aggressive blood cancer which is the most common type of non-Hodgkin lymphoma—follicular lymphoma, mantle cell lymphoma, and, most recently, multiple myeloma, according to the UPMC Hillman Cancer Center.
According to OHSU Knight Cancer Institute, CAR T cell therapy “is a particular breakthrough for children with leukemia, and for adults with certain hard-to-treat blood cancers,” but also holds promise for other diseases.