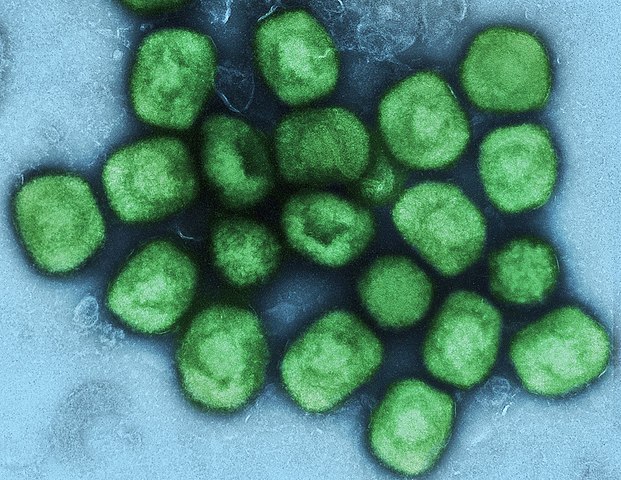
On Aug. 14, the World Health Organization (WHO) declared an international emergency as a highly virulent strain of mpox spreading from the Democratic Republic of the Congo helped drive a 22% mpox increase in the African region.
The declaration of a public health emergency of international concern, “the highest level of alarm under international health law,” will enable a campaign to provide vaccination and other measures to combat the clade I and clade II versions of the mpox virus, explained WHO Director-General Tedros Adhanom Ghebreyesus.
The more contagious and more deadly clade I virus has been a problem in the Democratic Republic of the Congo for more than a decade, with infection rates growing recently.
“Last year, reported cases increased significantly, and already the number of cases reported so far this year has exceeded last year’s total, with more than 14,000 cases and 524 deaths,” Ghebreyesus said.
A WHO report released Aug. 12 shows clade I has spread to four new countries that had not previously seen any mpox: Burundi, Kenya, Rwanda, and Uganda.
The WHO African Region reported 567 new clade I and clade II mpox cases in June, a 22% increase over the previous month.
The WHO last declared an international public emergency in July 2022 due to the spread of the clade II mpox virus. That outbreak, brought under control with the help of vaccination, caused nearly 100,000 cases in 116 countries and killed about 200 people.
Clade I is proving more deadly.
“DRC health authorities reported the case fatality rate (CFR) from suspected clade I mpox in 2023 was 4.6% and rose to 6.7% throughout early 2024; however, this may be an overestimation because of challenges in completeness of case reporting,” according to the U.S. Centers for Disease Control and Prevention (CDC). The fatality rate in the U.S. would probably be lower, given the availability of high-quality health care, noted the CDC.
The response and the vaccines
The public health emergency declaration came the day after mpox led to the first-ever emergency declaration by the Africa Centres for Disease Control and Prevention since its formation in 2016.
WHO announced a $15 million plan “to support surveillance, preparedness and response activities.” WHO Director-General Ghebreyesus said the organization is tapping into the WHO Contingency Fund for Emergencies and called for donations from other countries to help with the effort, including an expansive vaccination campaign.
Ghebreyesus “triggered the process for Emergency Use Listing for mpox vaccines, which will accelerate vaccine access for lower-income countries which have not yet issued their own national regulatory approval,” WHO said.
While existing mpox vaccines that are already approved by the U.S. Food and Drug Administration (FDA) are effective against clade I and clade II of the virus, officials have said there are nowhere near enough vaccines available in African countries to mount a meaningful vaccination campaign right now. Congolese authorities have requested 4 million doses of mpox vaccine, the Associated Press reported.
Bavarian Nordic, the maker of the Jynneos vaccine approved by the FDA for both mpox and smallpox, said it can provide 10 million doses of the vaccine to African countries by 2025, according to Bloomberg.
On Aug. 8, Bavarian Nordic received a $156.8 million order from the U.S. Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, to manufacture and store additional Jynneos vaccines, the company said.
In addition to vaccines, the FDA-approved treatment, TPOXX, an antiviral made by SIGA Technologies, can reduce hospitalization and mortality if mpox is contracted.
Bavarian Nordic and SIGA Technologies are members of the Biotechnology Innovation Organization (BIO).