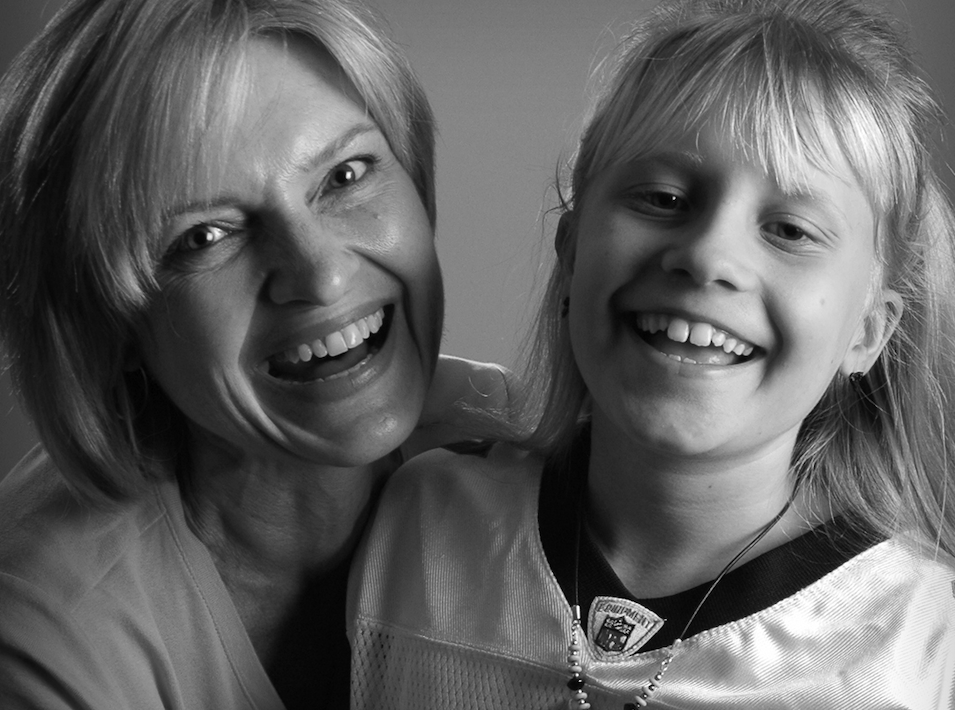Sharon King’s daughter Taylor was a happy surprise.
And up until Taylor was in the first grade, she was developing normally. But the first signs of trouble began after Taylor failed a standardized reading test.
“She just bottomed out on her reading scores,” King recalls, “which was odd, because she had been reading before she was in kindergarten. It was a real head-scratcher.” Taylor was later retested, and her scores improved, alleviating some of the concern for her family for the time being.
Early in second grade, there were signs of problems with Taylor’s vision. “First, she was having trouble seeing in low-light situations. Again, another head-scratcher,” said King. Before long, Sharon noticed that Taylor was having to sit very close to a screen, either at school or when watching television.
A pediatric ophthalmologist could find no evidence of visual issues. In the meantime, inexplicable symptoms continued to progress in Taylor, with no answer as to why. King was soon talking to teachers about the issue, eventually taking Taylor to the neurologist to try to find solutions. Was Taylor just a poor test taker? No. Was it ADHD? No, that wasn’t it, either.
Unbeknownst to Taylor and her family, they were dealing with a rare problem, yet paradoxically, a problem that many families have had to face in their own way. Taylor was experiencing the onset of a rare disease—specifically, an ultra-rare disease.
Individually rare, collectively large
Though this experience can be isolating for families, the Kings’ story is not all that unusual.
“The definition of a rare disease in the U.S. is that it has a patient population of under 200,000,” explains Dr. Courtney Silverthorn, Vice President of Strategic Alliances and Innovation at the Foundation for the National Institutes of Health (FNIH). “Some rare diseases that we work with are those with an even smaller patient population.”
As explained by FDA historian, John Swann, Ph.D., “By the early 1980s…‘rare diseases’ [collectively] affected 20-25 million patients who, together, suffered from approximately 5000 rare diseases—some of which affected as few as about a dozen individuals.”
While these diseases individually are rare, the collective community is actually quite large. Another problem with rare diseases is that, historically, those studying them have been disconnected—whether they were in academia, the clinical space, industry, or government. Luckily, today, with the internet and digital archiving, more and more people and organizations are able to connect to solve problems.
How FNIH is bringing the rare disease community together
Aimed at addressing some of healthcare’s most overlooked issues, the FNIH has used breakthroughs in technology and medical innovation, as well as a growing body of understanding, to develop programs aimed at pulling together resources that would otherwise stay disconnected.
The Bridge to Accelerating Gene Therapies: The Bespoke Gene Therapy Consortium
Programs like the Accelerating Medicines Partnership® (AMP®) Program, the Alzheimer’s Disease Neuroimaging Initiative (ADNI), the Biomarkers Consortium (BC), the Partnership For Accelerating Cancer Therapies (PACT), and more are moving the needle on a variety of diseases. And last 2021, the FNIH added the Bespoke Gene Therapy Consortium (BGTC) to the roster.
As Bio.News reported in May 2023, “The consortium aims to develop and advance gene therapies tailored to the unique genetic makeup of individuals with rare diseases, which affect more than 30 million people in the United States. By harnessing the power of gene editing and personalized medicine, the consortium aims to revolutionize the treatment landscape for these often-neglected conditions.”
“The consortium was looking for opportunities that first, didn’t have any current commercial interest, because we didn’t want the consortium to be a substitute for a company’s pipeline,” Dr. Silverthorn explains. “And we were also looking for opportunities to bring things to patients who would otherwise not have any therapeutic options, and so all of the BGTC patients that we’ve received tended to be those in the ultra-rare disease space.”
As part of its program, BGTC announced the selection of “eight rare diseases that will make up the clinical trial portfolio,” including:
- Charcot-Marie-Tooth disease type 4J
- Congenital hereditary endothelial dystrophy (CHED)
- Morquio A syndrome
- Multiple sulfatase deficiency (MSD)
- NPHP5 retinal degeneration
- Propionic acidemia (PCCB)
- Retinitis pigmentosa 45
- Spastic paraplegia 50 (SPG50)
These eight diseases toe a delicate line between having little to no commercial movement, while still having enough research and clinical data behind them to allow for future innovation development. The work runs the gamut from research to regulation to clinical trials, with many first-in-human clinical trials being facilitated by the consortium. No stone is left unturned and every aspect of the regulatory pathway is revealed.
In February 2024, BGTC released its first of several deliverables: a Regulatory Playbook for the development and regulatory submission of adeno-associated virus (AAV) gene therapies for rare diseases.
Playbooks such as these are important because many, if not most, of the companies and organizations working to solve rare diseases are small to mid-sized biotechs—even startups—meaning the risk researchers, investors, and clinicians take is much higher. By creating regulatory pathway documents, BGTC aims to shorten the process of beginning clinical trials for both patients and innovators.
“We can do better than that”
For rare disease families like the Kings, it took many months and a lot of work to finally figure out what was going on with Taylor.
“She was diagnosed in 2006, just before her eighth birthday, and in that time, I had seen a lot of change,” recalls King.
Taylor was diagnosed with CLN1 Batten disease, which is part of a small group of disorders that “affect the nervous system and typically cause worsening problems with vision, movement, and thinking ability.” Taylor’s disease was so rare that the known patient population is in the 30s, not the 1,000s or even 100s.
To say her disease was ultra-rare almost feels like an understatement; she had a disease so rare that her doctor said that not only was there no treatment for Taylor, but there wasn’t going to be any treatment anytime soon.
“There’s nothing you’re ever going to be able to do in her lifetime or yours,” Taylor’s doctor told King. “But I think we can do better than that. We are going to have to straighten these regulatory pathways and be willing to look at the smaller, very rare conditions in a new way.”
This force of conviction brought King into contact with BGTC and is shared by FNIH as well as the myriad partners the organization has brought together.
The first of many steps
“The Accelerating Medicines Partnerships® Bespoke Gene Therapy Consortium recognized the need for a comprehensive playbook that would serve as a guiding framework for the development and regulatory submission of adeno-associated virus (AAV) gene therapies for rare diseases,” the playbook’s prologue reads. “Building out this playbook to support the key processes up to a sponsor’s first-in-human (FIH) trial required a collaborative and modular approach. Version 1.0 of the playbook is designed to serve as a one-stop-shop guide and roadmap to investigational new drug (IND) submission for these innovative gene therapies.”
The consortium plans to continue to update versions of the playbook as their information and resources grow.
The science that the playbook addresses hints at the exciting work being facilitated and explored by the FNIH and its partners. AAV gene therapies easily sound like technology pulled from a sci-fi novel, but as our rapidly developing healthcare landscape is proving, the future is now.
Many rare diseases are caused by unhealthy genes in patients, a problem that often affects children and compounds over time. Put very (very) simply, AAV gene therapies use a virus (specifically the adeno-associated virus), which does not cause illness in humans, to almost infect patients with a cure.
“You can think of it as sort of a carrier for that healthy gene,” explains Dr. Silverthorn. “And then once the healthy gene is in the patient, it can take over the work of the defective gene.”
“It’s very different from what we would think of as the normal pharmaceutical model of drug development and drug treatment,” Dr. Silverthorn continues. “Instead of making a small molecule that might be delivered in a pill or a tablet or an injection, AAV gene therapy can be delivered where it is needed and is much more focused. Sometimes that focus is the whole body, but sometimes it is just in a specific area like the retina or in the spinal fluid.”
If the release of the current regulatory playbook is any hint, greater things are to come—and not a moment too soon, as many rare disease patients and their families cannot wait.
Committed to a solution
“Call me a cockeyed optimist, but I don’t believe in the impossible,” says King. “When we started Taylor’s journey, people came together with a common goal, common interest, and shared purpose. I believe that we will find a way, either through the development of platforms for conditions that can be treated in similar ways, or through the continued development of new medical innovation.”
While Taylor passed away in May 2019, her life has acted as an intense spark for her mother.
“Friends often come to me and say, ‘Sharon you know, Taylor has been gone five years. Aren’t you done? Don’t you want to play tennis?’ No,” she said. “I am committed. I have lived this experience, and I’m committed to seeing change and I believe wholeheartedly, we can make it happen.”
“My story is far from unique,” King asserts. “My experience started in 2006, and here in 2024, we’re still trying to find a solution. Luckily, we actually have a great public-private partnership with BGTC, who’re throwing their time and their resources at this challenge. We are working to get this done. It’s beyond time, and you know, as a mom I’m grateful. I’m grateful to every organization who says, let’s get this done.”
Featured image by Stacy Carter.